Mephedrone is classified as a “party drug” because of its psychostimulant and empathogenic effects, which are comparable to those brought about by the use of cocaine, methamphetamine, and/or MDMA. Mephedrone really has several molecular and neurobehavioral effects with substituted amphetamines because of their structural similarity. Motbey noted the mephedrone-induced increase in Fos expression in the supraoptic nucleus, brain, striatum, and ventral tegmental region. These brain areas are also seen to be activated following exposure to MDMA and/or methamphetamine. Since mephedrone rapidly penetrates the blood-brain barrier, users have described pleasant and quick but fleeting feelings of exhilaration, happiness, need to communicate, reduced aggressiveness, higher music enjoyment, increased libido, improved alertness, and focus. These positive effects usually appear 15–45 min after oral administration of mephedrone (or a few min after nasal insufflations), and they last only ca. 2–3 h, which encourage mephedrone users to repeat the dose. Therefore, even though a normal dose of mephedrone is between 100 and 200 mg, a user may ingest up to 4 g of the drug in a single session.
Mephedrone’s beneficial effects might be accompanied with unpleasant acute side effects as dilated pupils, impaired vision, time dilation, or hot flushes. Long-term mephedrone usage may have negative psychological, renal (kidney damage), musculoskeletal (bruxism), skeletal, and cardiovascular effects, including depression, panic attacks, agitation, paranoia, auditory and visual hallucinations, and self-mutilation. The mechanism of action of mephedrone is not yet fully understood. The three main monoamine transporters (serotonin transporter SERT, dopamine transporter DAT, and noradrenaline transporter NET), as well as serotonin receptors 5-HT1A and 5-HT2A, omega-1 receptors, and α1- and α2-adrenoreceptors are the sites of mephedrone application to produce similar effects. Mephedrone works as a substrate of SERT, DAT, and NET, causing the release of the corresponding monoamines into the synaptic cleft and preventing their reuptake, as shown by a number of independent studies.
There aren’t many articles describing mephedrone’s effects on the brain’s dopaminergic nuclei. As previously mentioned, Motbey found that mephedrone treatment to adolescent Wistar rats activated the caudate-putamen and the ventral tegmental region. The nucleus accumbens was stimulated by mephedrone as well. In other research, rats exposed to various mephedrone dosages showed increased levels of dopamine and/or serotonin in the nucellus accumbens. Additionally, Grochecki demonstrated a number of alterations in the hippocampus of adolescent rats that could be seen following the administration of mephedrone, including increased levels of matrix metalloprotease (MMP)-9, up-regulation of the NMDA receptor subunit GluN2B, and decreased levels of postsynaptic density protein (PSD)-95. When mephedrone was given to pregnant female mice, Naseri et al. found that the offspring had hippocampus injury. Though Angoa-Pérez and colleagues did not observe any impairments in hippocampal serotonin nerve endings in female mice given mephedrone. The disparity between these two studies may be attributable to different study designs, notably in the tested dosages; in the latter research team, mephedrone was utilized at a substantially greater dose.
Mephedrone is utilized on a global scale by both people and lab animals, although not all of its qualities have been fully uncovered. There aren’t many studies from behavioral trials relating to the main effects of mephedrone in the literature that is currently available. Moreover, many discrepancies are found between the outcomes described in published manuscripts, which suggest that further research is needed. We are certain that the results of this study will help us comprehend the biological impacts of mephedrone. We sought to show some new qualities of mephedrone while also advancing general information about the substance’s action in living things when we designed the current study. We are certain that the results of this study will help us comprehend the biological impacts of mephedrone.
Methods and materials we used in the study
We used male mice weighing 20-25 grams as test animals. Pure mephedrone hydrochloride (dose 0.05, 0.125, 0.25, 0.5, 1, 2, 5 mg/kg) and pentylentetrazol were used as control psychoactive substances. These two substances were dissolved in saline and injected intraperitoneally. In our experiment, we used the following behavioral tests:
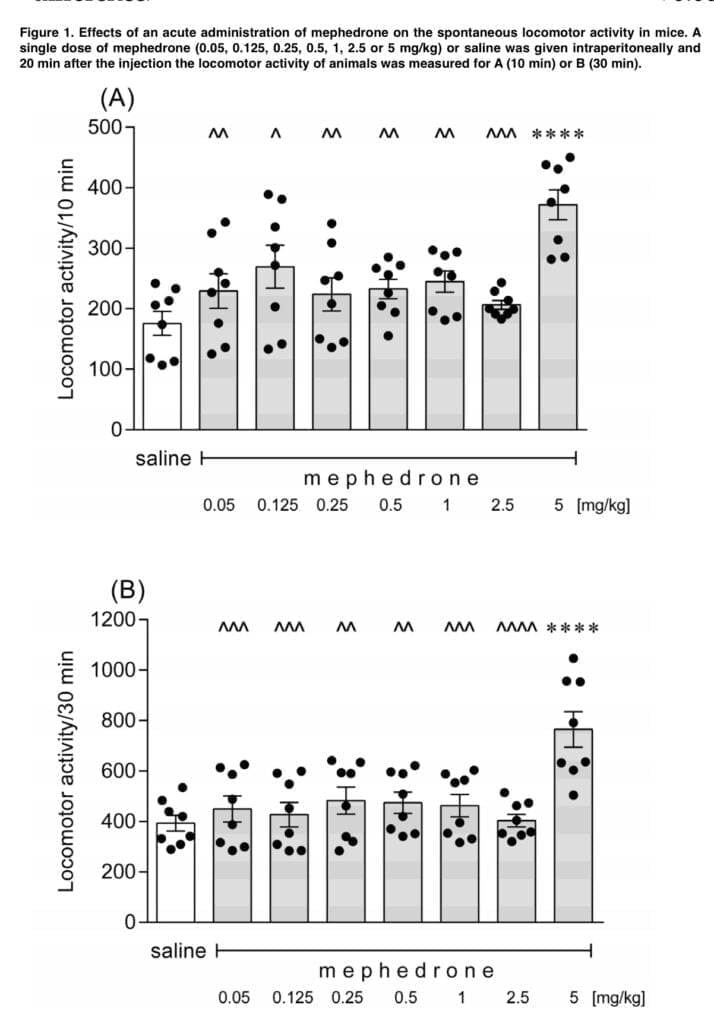
- Measurement of the spontaneous locomotor activity – the locomotor activity of each mouse was recorded using a photocell apparatus which was placed in a sound- attenuated experimental room. Animals were caged 20 minutes after mephedrone injection in the dose range from 0.05 to 5 mg/kg. Locomotor activity was assessed at 10 and 30 minutes.
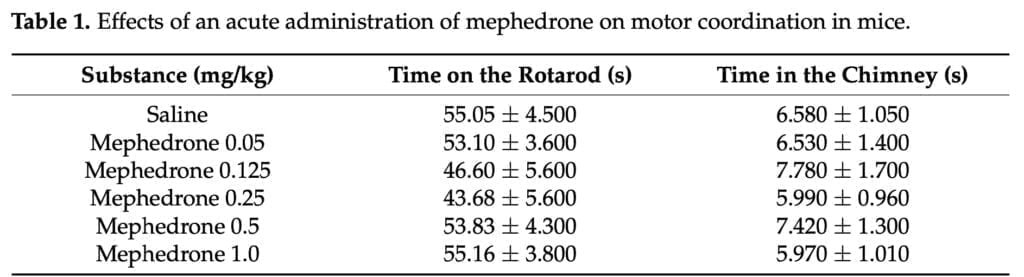
- Rotarod tests – mice were trained daily for 3 days on a bar (2 cm in diameter) rotating at a constant speed of 18 rpm. Mice were placed on a rotating rod 20 min after mephedrone (0.125, 0.25, 0.5 or 1 mg/kg) or saline i.p. injection. The experimental session lasted for 60 s. The time spent on the rotating rod by a given mouse was measured.
- Chimney test – animals had to climb backward up a plastic tube (3 cm in inner diameter, 25 cm long). Mice were placed in the chimney 20 min after mephedrone or saline i.p. injection. The experimental session lasted for 60 s. The time spent in the chimney by a given mouse was measured.
- Elevated plus maze – the test device was a black Plexiglas maze with two open arms, two equal-sized enclosed arms, and two oppositely placed open arms. A weak light lit the maze, which was elevated to a height of 50 cm above the ground. A particular mouse was positioned in the center of the labyrinth, facing the closed arm, for a total of 20 minutes after receiving an intraperitoneal injection of mephedrone or saline. The mouse was then free to roam the maze for 5 minutes. The number of entries into open arms and the amount of time spent in open arms were used to gauge the anxiogenic/anxiolytic potential, whereas the number of entries into closed arms and the sum of all entries into either kind of arm were used to gauge the animals’ overall motor activity in the EPM.
- Modified EPM (mEPM) – a dark Plexiglas labyrinth that was 50 cm above the ground served as the testing equipment. It had a center platform, two open arms, and two enclosed arms that were in opposition to one another. It was formed like a “plus” symbol. A total of 20 min after mephedrone or saline i.p. injection, a given mouse was placed in the distal end of an open arm, facing from the central platform (the acquisition session). Transfer latency, or TL1, was used to gauge how long it took the animal to go to one of the enclosing arms. The mouse was placed in an enclosed arm and given a further 60 seconds to explore the maze if it didn’t enter one after 90 seconds had passed.
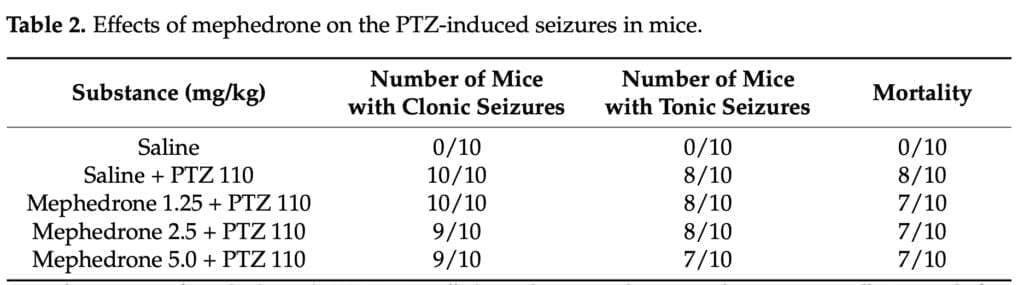
- Pentylenetetrazol (PTZ) seizure test – I.p. administration of mephedrone or saline was given 20 minutes before the s.c. injection of PTZ (110 mg/kg). Animals were moved to an open field (50 cm in diameter) immediately following PTZ administration, and they were monitored for 60 minutes for any instances of clonic seizures, tonic convulsions, or death.
Analysis and discussion of the experimental data obtained
In line with expectations, our study found that acute intraperitoneal injections of mephedrone led to dose-dependent hyperactivity in mice. Mephedrone’s effective dosage was 5 mg/kg, whereas ineffective doses ranged from 0.05 to 2.5 mg/kg. These results are consistent with those of other authors who showed that mephedrone increased spontaneous locomotion in a variety of murine and rat strains at doses between 3 and 40 mg/kg, following intraperitoneal, intravenous, oral, and subcutaneous administration as well as acute, repeated “binge-style,” intermittent exposure. The severity of the mephedrone-induced hyperlocomotion may be equivalent to that seen after taking the same amount of MDMA, although it may be lesser than that seen after taking amphetamine, according to the literature data. The functional observational battery (FOB) carried out by Marusich in ICR mice revealed that the mephedrone-related activation of the spontaneous locomotion in animals may be accompanied by general stimulation, increased stereotyped head weaving and increased head circling. However, Huang found that mephedrone treatment at a level of 5.6 mg/kg resulted in a dose-dependent reduction in wheel running in Wistar rats. Such an animal reaction was comparable to that seen after receiving MDMA (5.6 mg/kg). However, the mephedrone-induced ambulatory hyperactivity appears to be independent of both the circadian cycle and the surrounding temperature.
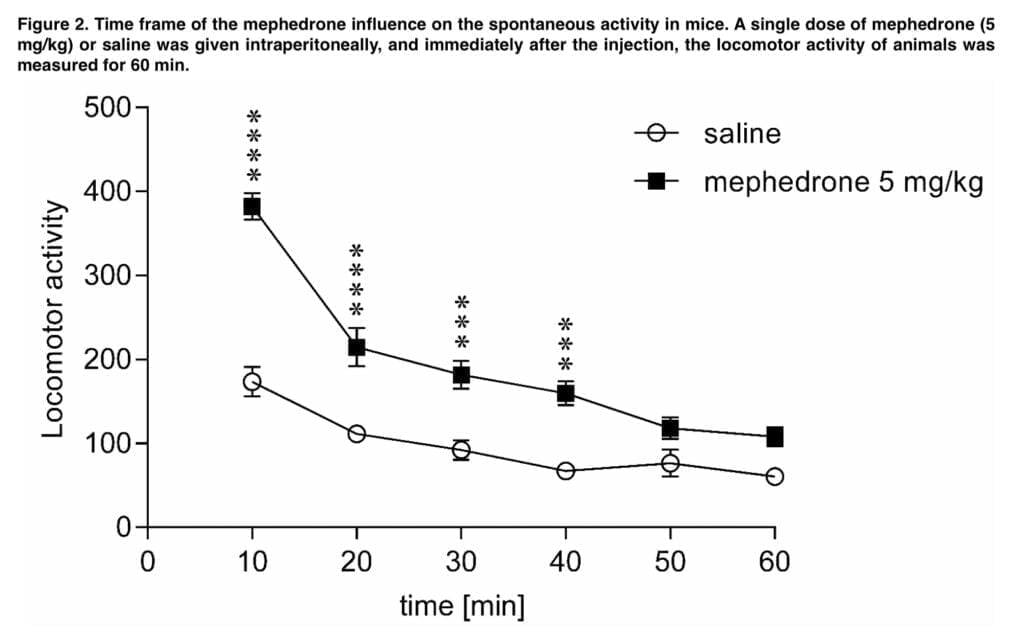
Our additional research revealed that mephedrone readily penetrates the blood-brain barrier and swiftly reaches its maximal concentration in the brain following exposure, since the effects on spontaneous locomotor activity were most prominent within the next 10 minutes.
The motor activity of the tested mice recovered to the control levels 50 min following the injection (5 mg/kg), demonstrating that these effects were temporary and only persisted for a brief period of time. Similar findings were made by other writers, including Kehr and Shortall, who observed that the mephedrone-induced ambulatory hyperactivity is not a long-term effect and that it goes away sooner than the motor stimulation seen after amphetamine and MDMA exposure, respectively. Users of mephedrone report that the drug’s intended effects generally begin to manifest 15–45 minutes after oral administration and a few minutes after nasal insufflation, although they are fleeting and pass away within 2-3 hours. In addition, the duration of the intended effects following intravenous mephedrone treatment is significantly shorter, lasting just 30 minutes. This explains why users of mephedrone rapidly redose the drug, much as is seen with cocaine. Most probably, the mephedrone-induced increase in mobility is due to alterations in the serotonergic and dopaminergic neurotransmissions since antagonism of the serotonin 5- HT1A, 5-HT1B, and 5-HT2 receptors with WAY-100635, GR 127935, and ketanserin respectively. Interestingly, neither 6-hydroxytryamine nor SB-258719 changed the effects of mephedrone; instead, sulpiride potentiated the increase in ambulatory activity caused by mephedrone. Mayer demonstrated that bioactive metabolites of mephedrone contribute to behavioral stimulatory effects of the drug.
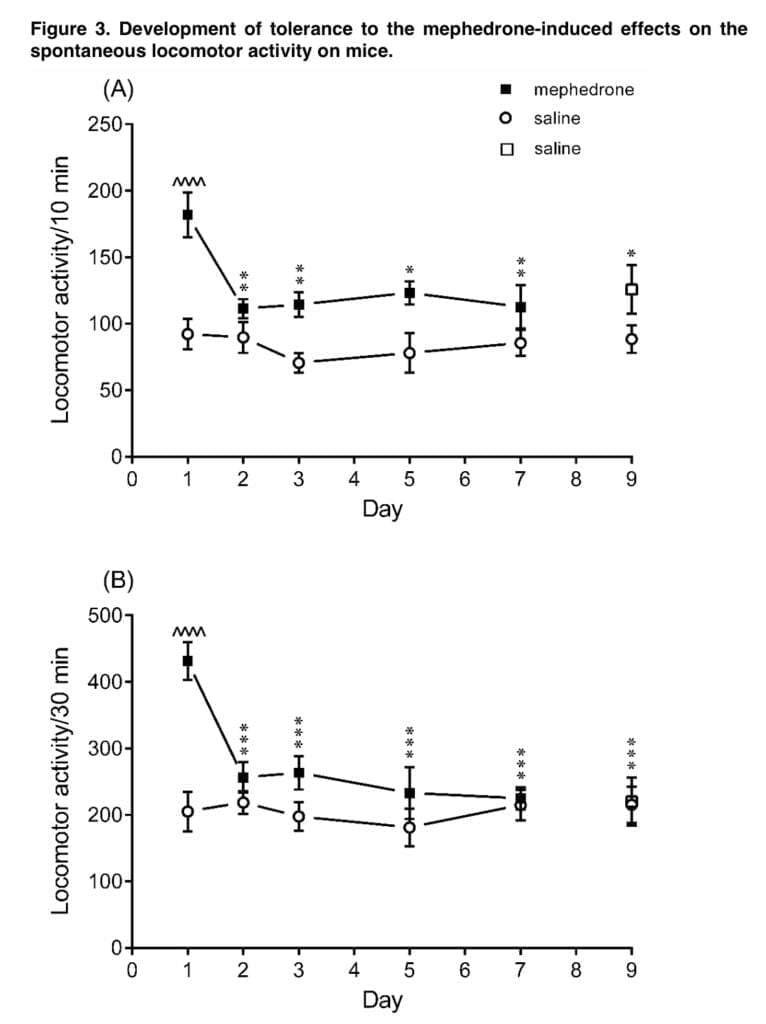
Another set of our studies shown that administering mephedrone (5 mg/kg) over a period of days may lead to the development of tolerance to the ambulatory hyperactivity brought on by mephedrone. Medical literature defines drug tolerance as a progressive loss of an agent’s effectiveness after repeated administration. Actually, mephedrone did not make the mice more mobile after the second intraperitoneal dose, and the same pattern was seen with successive injections. However, after stopping the mephedrone therapy, we did not see any withdrawal symptoms developing. Only a few reports have been made, as far as we are aware, of animals becoming tolerant to the effects mephedrone causes. The data from the literature suggests that tolerance to mephedrone’s effects after repeated use may also be seen in people. However, the “binge-style” administration of mephedrone in the Shortall research resulted in consistent hyperactivity after each dosage, with a rapid start and brief duration.
Two well-known behavioral paradigms, the rotarod and the chimney tests, are employed in preclinical research to assess a drug’s potential to impair rodent motor coordination. A reduction in the amount of time spent on the rotarod or the mice’s failure to climb back up the tube within 60 minutes are signs of motor impairment. In consequence, when motor incoordination is observed only in the rotarod test, it can be assumed that a given drug exerts depressive effects on the central nervous system. However, a particular medication most likely possesses myorelaxant potential if motor incoordination is seen un both tests. In the current investigation, none of the mephedrone dosages examined disrupted the animals’ motor coordination or caused myorelaxation. It was consistent with Marusich’s findings, who discovered that mephedrone did not impact ICR mice’s behavior on the rotarod when administered within the measured dosage range of 3-56 mg/kg (i.p.).
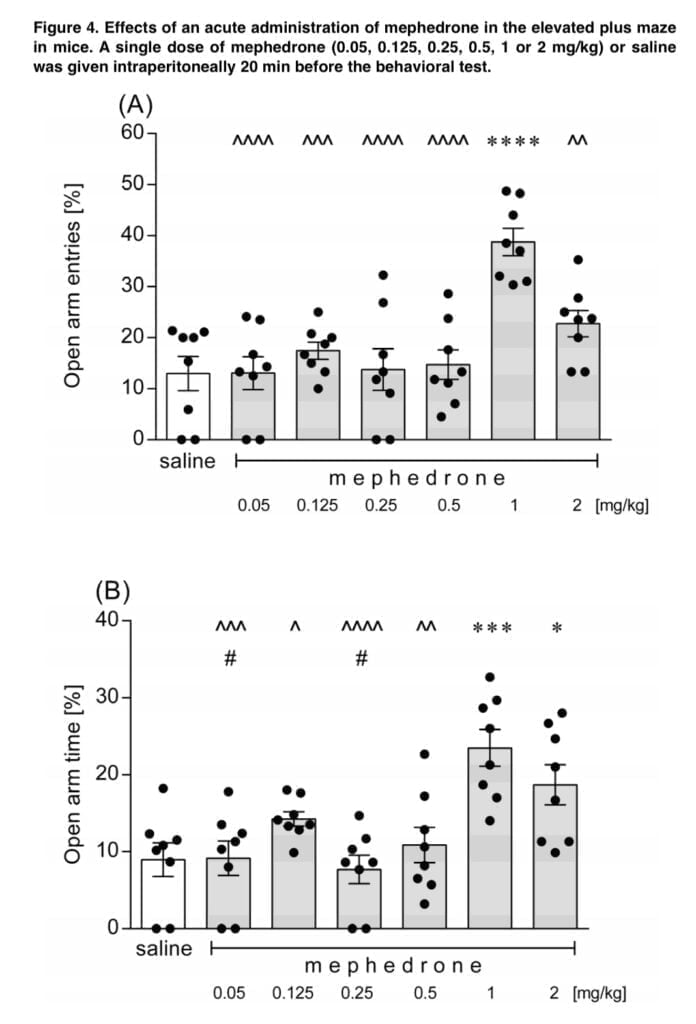
Animal behavior in the EMP test is described on the newborn conflict between preference for safe places and the natural motivation to explore new areas, even if they are potentially uncomfortable. Thus, the anxiogenic-like effect is indicated by a decreased number of entries to the open arms of the maze and by the shorter time spent in these arms. Mephedrone demonstrated a dose-dependent anxiolytic potential in the current investigation. Only 1 mg/kg of mephedrone considerably increased the proportion of animals entering the open arms, compared to the two highest dosages of the drug used in the experiment, which were 1 and 2 mg/kg. Lower mephedrone dosages (2.5 and 5 mg/kg, s.c.) resulted in increased exploration in the central zones, indicating a lowered degree of anxiety, whereas the highest tested dose of the substance (i.e., 20 mg/kg), resulted in more severe thigmotaxis, indicating an enhanced level of anxiety. Low dosages of mephedrone were used in our trials. Motbey also noted the thigmotaxic effects of mephedrone (15 and 30 mg/kg, i.p.) in Wistar rats. In actuality, anxiousness is one of the unfavorable effects associated with mephedrone use in people.
The PTZ seizure model was employed in the current investigation to determine if mephedrone has proconvulsant or anticonvulsant properties. None of the mephedrone doses used in our study protected mice from the PTZ-induced clonic seizures, tonic seizures, or mortality. However, the findings did not support mephedrone’s proconvulsant potential at the tested levels, which was consistent with the findings of Marusich and colleagues. This cathinone did not cause tremors or convulsions in the stated study, which used ICR mice, despite the fact that far greater dosages (up to 56 mg/kg) than the ones used by us were delivered intravenously. Human-based research on the neurotoxic effects is scarce, but according to the published records, tremors, convulsions, and self-limiting pre-hospital seizures have been mentioned among the mephedrone-related adverse reactions. Martinez-series Clemente’s of tests showed that mephedrone can cause short-term dopaminergic and serotoninergic neurotoxicity, although a large body of research suggests that these effects are rare and short-lived when mephedrone is taken alone. However, this cathinone may amplify the toxic effects of other illicit substances toward the monoaminergic systems when taken concurrently.
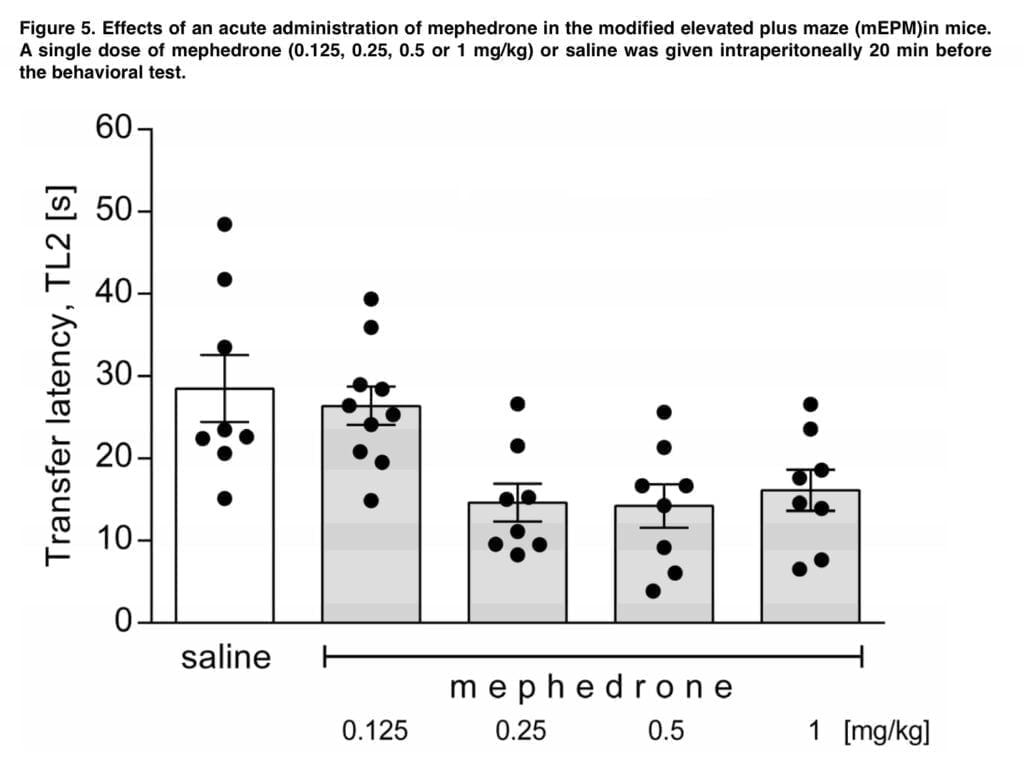
Mephedrone was given 20 minutes before the acquisition session (in both tests) in our trials, and its effects on memory acquisition were examined at the retention/trial session. Results from the mEPM and NOR tests indicate that mephedrone has a dose-dependent improvement in spatial memory but no effect on recognition memory. While the lowest administered dose (0.125 mg/kg) had no impact, the higher tested doses of the medication significantly reduced transfer latency during the retention session in the mEPM. Our findings are at odds with those made by Motbey, who emphasized the detrimental effects of mephedrone treatment on the ability to recognize unfamiliar objects. Den Hollander reported that mephedrone reduces working memory performance in the T-maze in C57BL/J6 mice. Wright discovered that this cathinone may increase visuospatial associative memory in rhesus macaques, but it has no effect on spatial working memory. Shortall noticed that it also inhibits retention of fear motivated contextual memory in Lister hooded rats. Human-based studies have shown that mephedrone consumption may reduce cognitive function and acute working memory. Among the negative side effects of mephedrone usage that might affect the central nervous system include memory loss and lack of attention. MMP-2 and MMP-9, which are found in several brain areas, are most likely responsible for mephedrone’s action toward memory functions. The effects of mephedrone on learning are not definitive and rely on a number of variables, such as the age, species, and dosage regimen of the investigated animals.
Conclusion
In conclusion, the main discoveries of the present investigation advance our understanding of the main impacts of mephedrone. In adult albino Swiss mice, we demonstrated that acute administration of this medication had no impact on motor coordination, may have anxiolytic potential, does not cause proconvulsant or anticonvulsant effects, does not alter recognition memory, but may enhance spatial memory. The use of different animal strains and tested dosages, as well as variations in lab settings, project designs, or experiment length, may account for discrepancies between our study’s findings and observations reported by other authors in regard to the EMP and the NOR test. We also showed that mephedrone-induced ambulatory hyperactivity is quick and transient, and that repeated treatment of this cathinone may result in the tolerance building up to these stimulatory effects.
